Explain How a Nuclear Reaction Differs From a Chemical Reaction
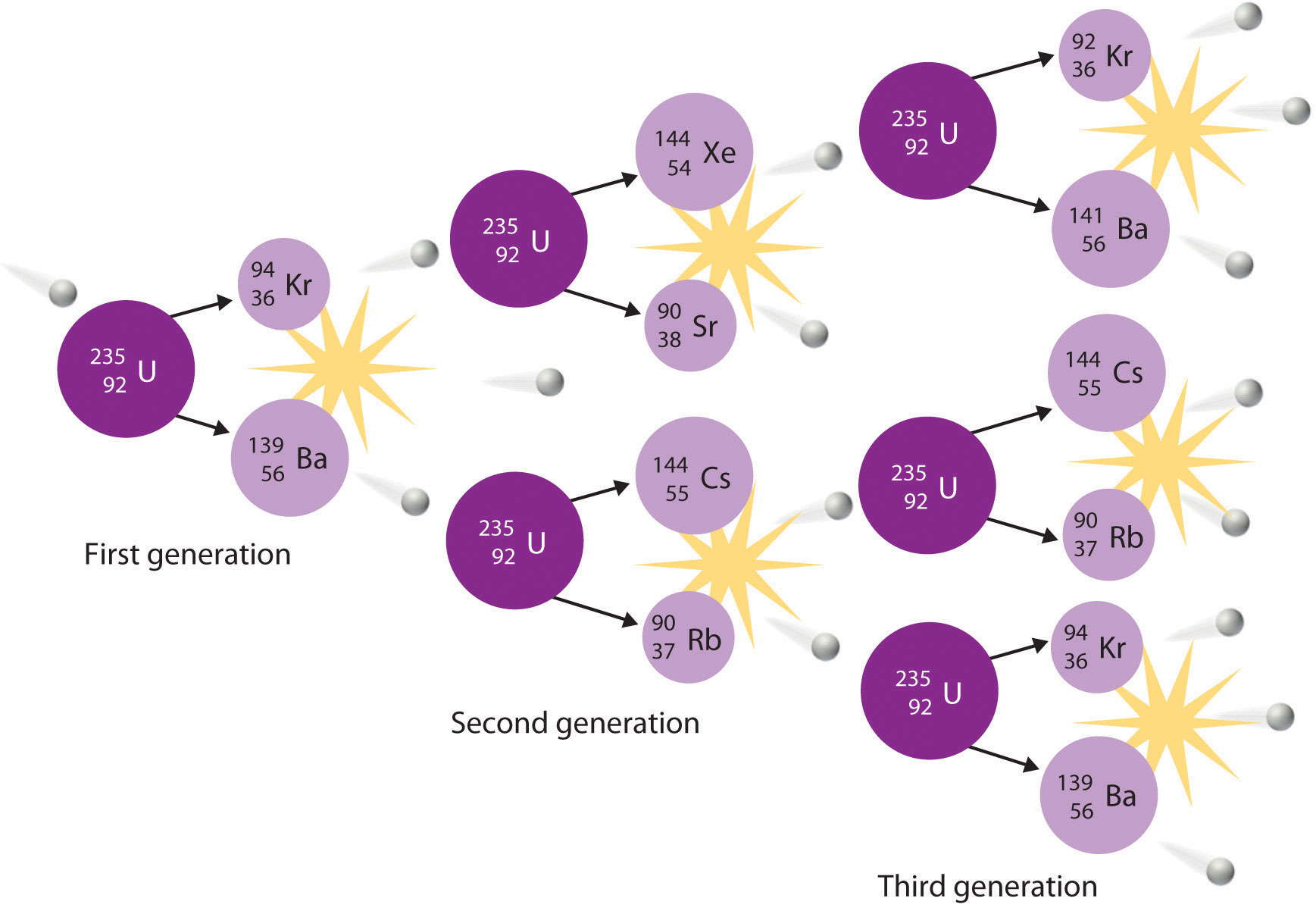
Type of Radioactivity Symbol of Emitted Particle nuclear symbol Charge of Particle Mass Number of Particle 3. Difference in mass is converted to energy via Emc 2 chain reactions require high enough concentration neutrons released as decay product each release triggers another decay continue until stable element in middle of periodic table like iron is reached Fusion.

What S The Difference Between Nuclear Fission And Fusion
Decay of Radioactive Elements.

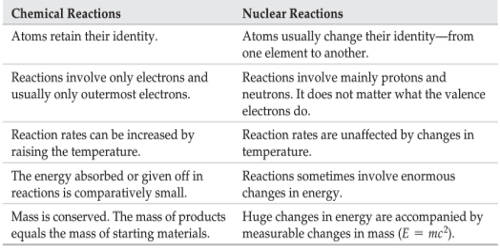
. Nuclear reactions are those reactions in which nuclear transformation takes place. An atom contains a nucleus. An atom has the same number of protons and electrons.
Nuclear reactions involve the decomposition of the nucleus and have nothing to do with the electrons. Nuclear reactions are processes in which one or more nuclides are produced from the collisions between two atomic nuclei or one atomic nucleus and a subatomic particle. It is electrically neutral.
Chemical reactions on the other hand involve only a rearrangement of electrons and do not involve changes in the nuclei. Distinguish among alpha beta and gamma radiation on. If a nucleus interacts with another nucleus or particle and they then separate without changing the nature.
In Chemical reactions it is the electrons in the outer shells that take part in the reaction. While nuclear reaction takes place in the atoms nucleus the electrons in the atom are responsible for Chemical reactions. In chemical reactions the energy is expressed in terms of kilojoules per mole.
The energy changes in any chemical reaction is very much less when compared with nuclear reaction. Nuclear vs chemical reactions a. No new element is.
List the four types of radioactivity in the first column and fill in the table below. While typical chemical reactions release energies on the order of a few eVs eg. In a nuclear reaction there is a change in the atoms nucleus.
The binding energy of the electron to hydrogen is 136 eV nuclear fission reactions typically release energies on the order of hundreds of millions of eVs. Nuclear chain reactions can be found mainly regarding nuclear fission reactions. Nuclear reactions are reactions where the nuclei of atoms take part in the reaction.
Another difference is the amount of energy required. The neutron absorption reaction is the most important type of reactions that take place in a nuclear reactorThe absorption reactions are reactions where the neutron is completely absorbed and the compound nucleus is formedThis is a very important feature because the mode of decay of such a compound nucleus does not depend on how the compound nucleus was formed. Briefly explain the difference between a nuclear reaction and a chemical reaction.
An element becomes a different element. A large unstable nucleus is split apart into two smaller nucleus man-made reaction 3. A nuclear transformation could be any of the following.
A nuclear reaction emits radiation changes the nucleus and stabilizes the proton and neutrons. An unstable atom loses its energy spontaneously through the ratio of the nucleus. While a chemical reaction requires or gives off a relatively small amount of energy through the breaking and forming of atomic bonds a nuclear reaction gives off a large amount of energy through the splitting fission or fusing fision of atomic neclei.
A nuclear reaction involves the protons and neutrons and chemical reactions involve the electrons. Nuclear fusion involves the combination of two light nuclei to form a heavier nucleus with emission of energy. Two small nuclei fuse to make a larger more stable nucleus - man-made reaction 2.
In nuclear physics and nuclear chemistry a nuclear reaction is a process in which two nuclei or a nucleus and an external subatomic particle collide to produce one or more new nuclidesThus a nuclear reaction must cause a transformation of at least one nuclide to another. A chemical reaction is where the atoms of one or more substances are rearranged to form a different substance. A chemical reaction is balanced in terms of mass only.
Alpha decay A nuclear reaction like α decay takes place in the nucleus of an atom. 1 Nuclear reactions involve a change in an atoms nucleus usually producing a different element along with the emission of radiations like αβ and γ etc rays. Chemical reaction Na Cl Na Cl A chemical reaction like the formation of NaCl involves rearranging the electrons which are outside the nucleus.
A nuclear reaction involves the atoms nucleus and a chemical reaction is the rearrangement of electrons. 6 rows The main difference between chemical reaction and nuclear reaction is based on how or where. Chemical reactions result only in the transfer of electrons But In Nuclear reactions the the nucleus in the reacting element and the produced element are not same Physics Science.
In a molecule atoms more or less share these peripheral electrons. Peripheral electrons play a major role in chemical reactions. A large unstable nucleus gives off an alpha particle He nucleus to become a slightly smaller but more.
Nuclear fission is the breakdown of an unstable atomic nucleus. Chemical reactions help us understand the properties of matter. If the two numbers differ the atom is said to be ionized but it retains the chemical nature determined by its protons.
Two notable types of nuclear reactions are nuclear fission reactions and. Chemical reactions involve the electrons in an atom whereas nuclear reactions involve the nucleus Chemistry Review and Terminology Lets do a little review of chemistry. The nuclides produced from nuclear reactions are different from the reacting nuclei commonly referred to as the parent nuclei.
Chemical reactions are how new forms of matter are made. Elemental Composition The elemental composition changes during a nuclear reaction resulting different types of nuclides than at the beginning. Explain what is happening in each of them there are at least four.
Lighter elements converted to heavier elements as lighter nuclei merge. A chemical reaction is the process in which reactants react chemically and convert into products by chemical transformation. The difference between controlled and uncontrolled chain reactions is that controlled chain reactions do not lead to any explosive effects whereas uncontrolled chain reactions lead to explosive energy release.
A nuclear reaction equation is a representation of the change that takes place as one nucleus is converted to another. The chemical reactions involve the transfer loss gain and sharing of electrons and nothing takes place in the nucleus. While nuclear reactions also may produce new matter nearly all the substances you encounter in daily life are the result of chemical changes.
Complete the following nuclear equations by providing the missing. During chemical reactions elements remain the same and do not lose their identity.

Fission Vs Fusion Physics And Mathematics Physics Notes Nuclear Physics

What S The Difference Between Nuclear Fission And Fusion

Nuclear Fusion Development Processes Equations Facts Britannica
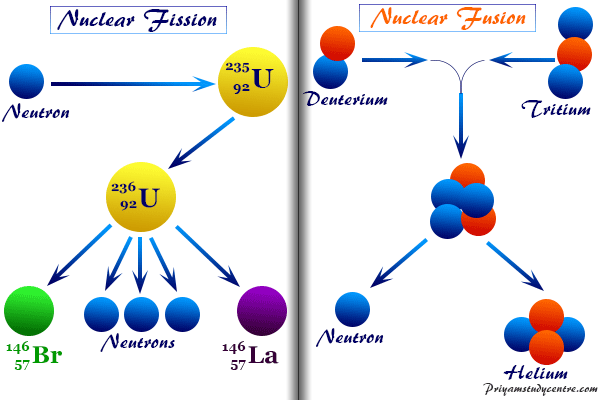
Nuclear Reaction Types Definition Examples
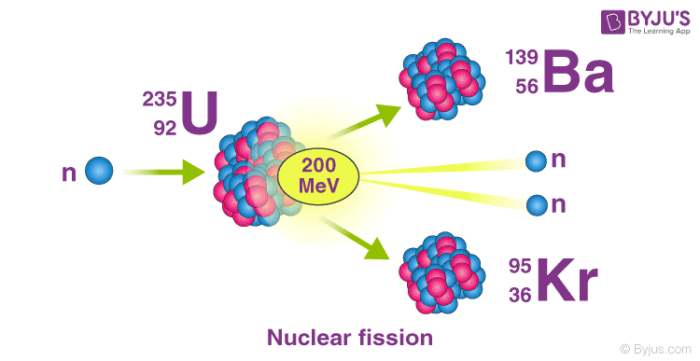
Nuclear Reaction Definition Types Examples With Equations
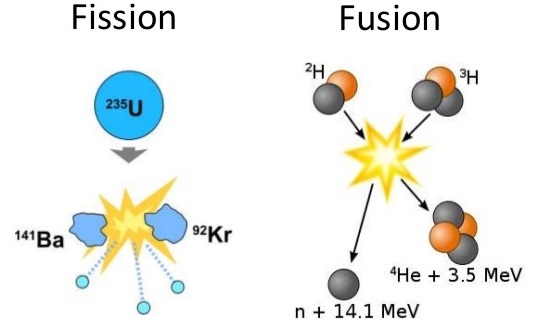
2 6 Fission And Fusion Chemistry Libretexts
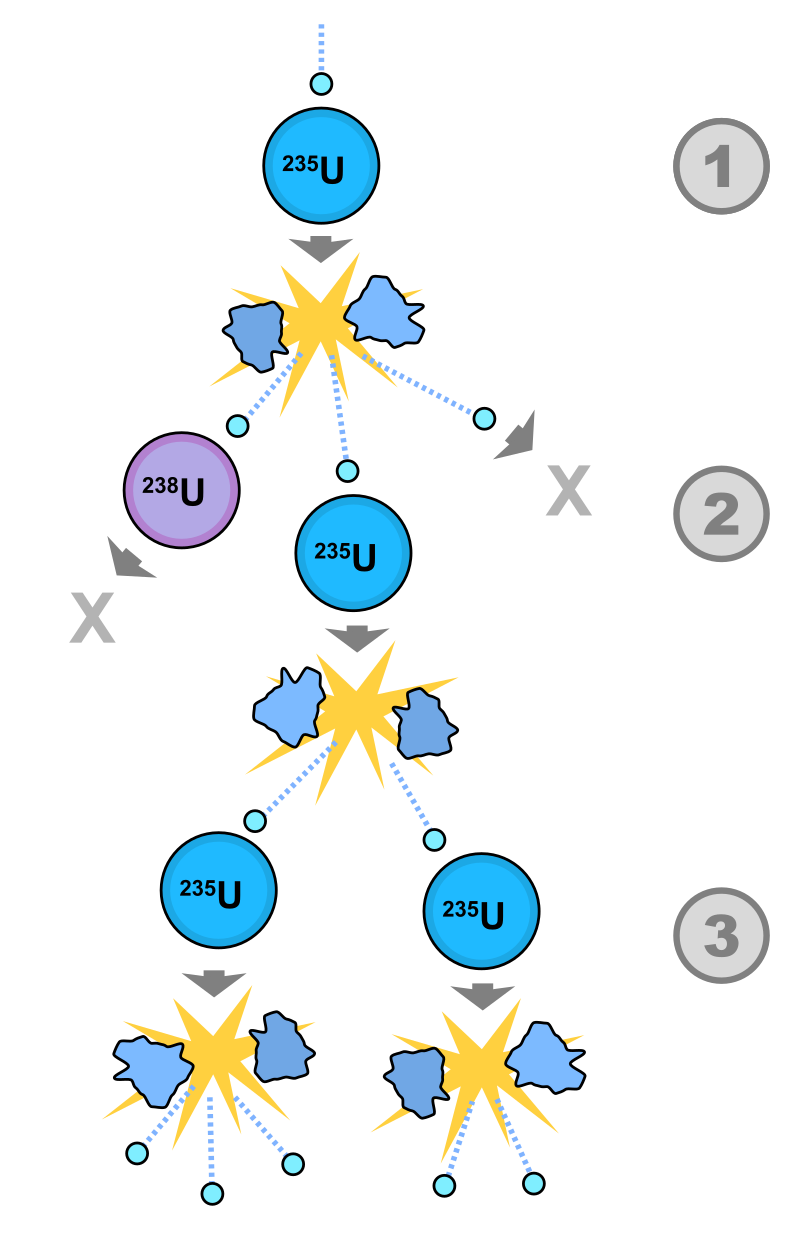
Nuclear Chain Reaction Energy Education
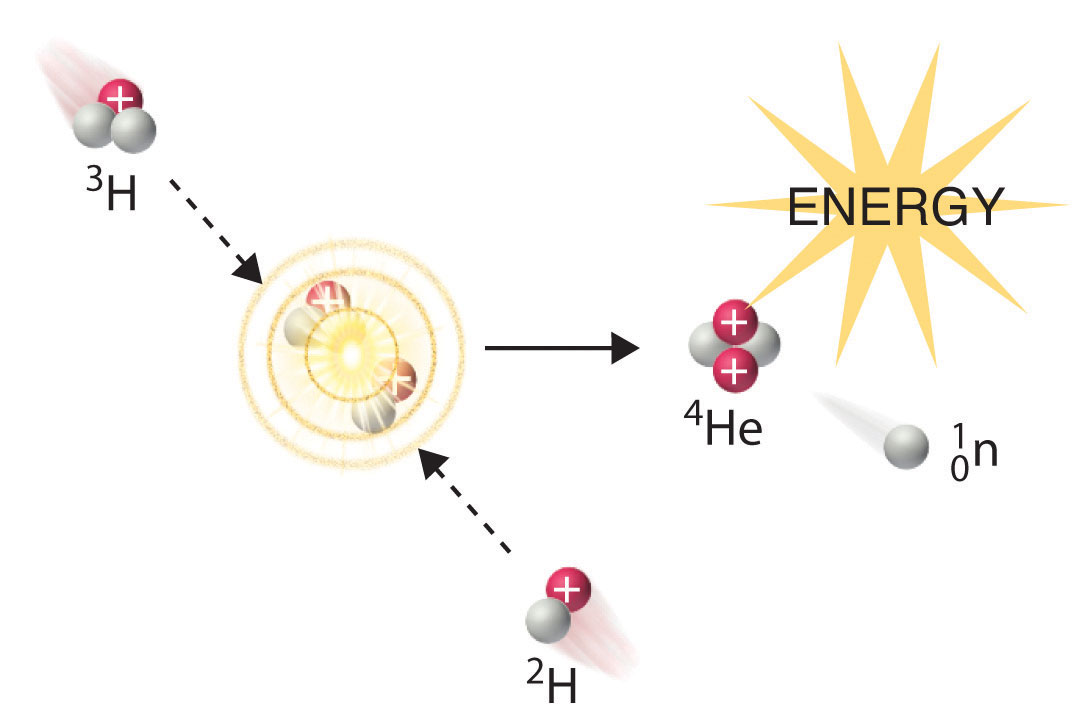
Fission And Fusion Chemistry Libretexts

What S The Difference Between Chemical Reactions And Nuclear Reactions And What Does That Mean For Isotopes Ch Nuclear Reaction Chemical Reactions Chemistry
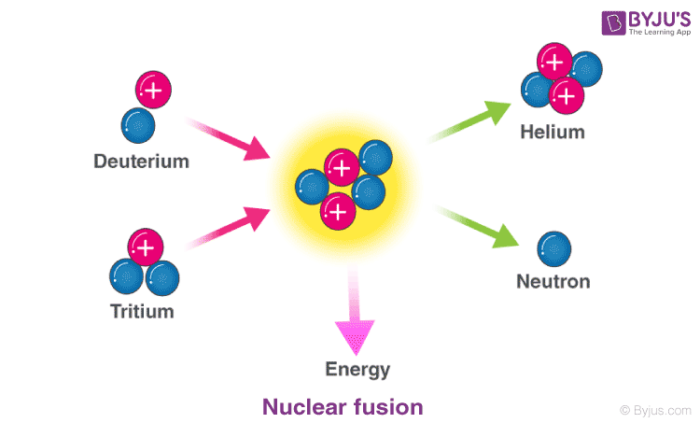
Nuclear Reaction Definition Types Examples With Equations

Nuclear Reactions Boundless Chemistry

Nuclear Energy Definition Sources Uses Facts Britannica
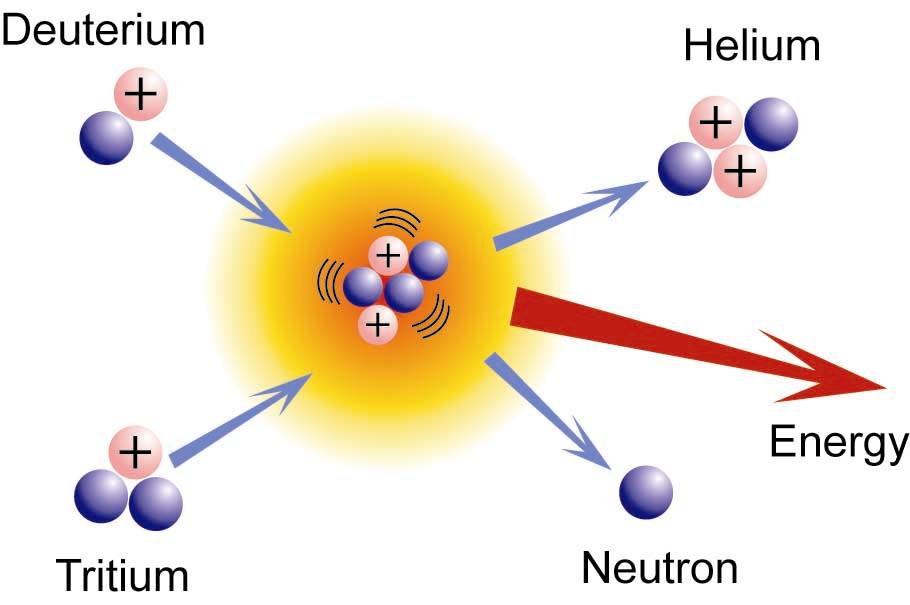
Fission And Fusion Chemistry Libretexts
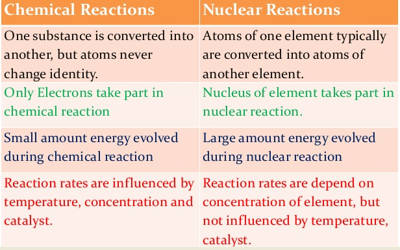
Difference Between Chemical Reactions And Nuclear Reactions Qs Study

What S The Difference Between Nuclear Fission And Fusion
Difference Between Chemical Reactions And Nuclear Reactions Qs Study

Radioactive Decay Types Article Article Khan Academy
Comments
Post a Comment